BCT-100 in Arginine Auxotrophic Cancers
Arginine and Arginine Auxotrophic Cancers
Arginine is a precursor of an array of biomolecules that are crucial for wide range of cellular functions. While arginine is not classified as “essential” amino acid, it is indeed indispensable in early life stages and in anabolic situations (e.g., growth spurs) and hyper-catabolic states (e.g., acute infections).
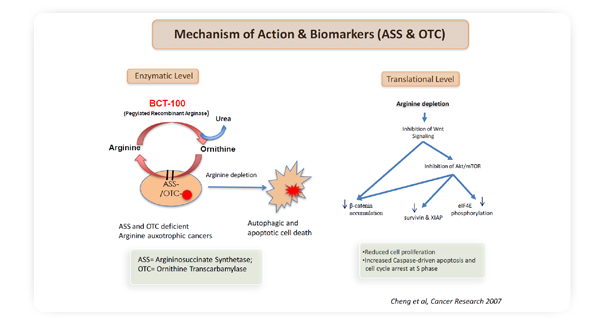
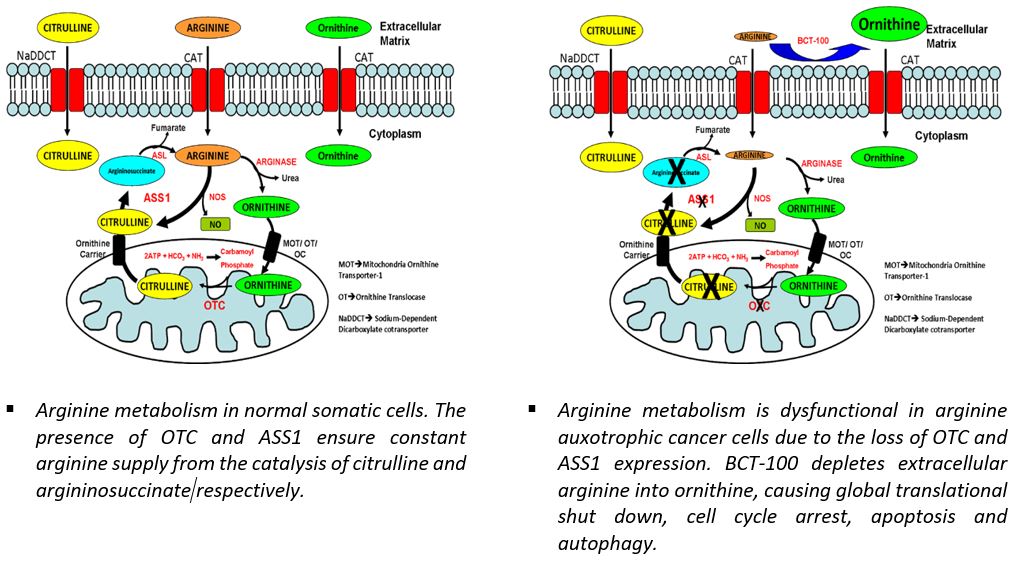
Arginine has been implicated in tumorigenesis in a number of research studies with tumor xenograft mouse models: tumors proliferated and metastasized when mice were fed with arginine; however, shrank and failed to metastasize when depleted of dietary arginine. For arginine auxotrophic tumors, arginine is an essential amino acid, depletion of which leads to cell death. In time of deficiency, normal cells can synthesize arginine from citrulline with a rate-limiting enzyme in the arginine production pathway, argininosuccinate synthetase 1 (ASS1). Cells lacking or under-expressing ASS1 die in the absence of exogenous arginine, because of their inability to synthesize arginine from citrulline. Similar to the loss of ASS1, dysfunctional ornithine transcarbamylase (OTC) can also have drastic effects on tumor cell survival upon arginine deprivation as the cells are not able to synthesize citrulline from ornithine, resulting in significant loss in citrulline supply for ASS1 to produce arginine.
BCT-100 in Arginine Auxotrophic Cancers
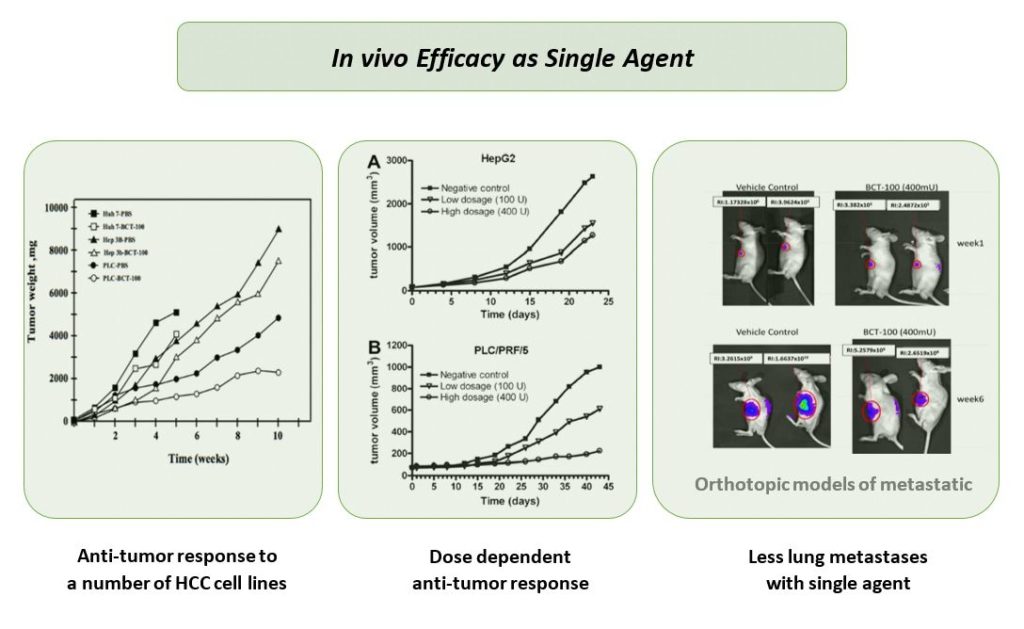
BCT-100 functions as an anti-tumor agent by systemically depleting arginine, thereby cutting off the arginine supply to tumor. Extensive pre-clinical studies have well proved BCT-100’s anti-tumor efficacy in wide range of cancer cell lines and tumor xenografts in nude mice.
At the molecular level, BCT-100 treatment resulted in inhibition of the Wnt/β-catenin and mTOR pathways and some of their downstream signaling effectors, including the anti-apoptotic molecules XIAP and survivin. Arginine auxotrophic cancer cells are highly dependent on the deregulated mTOR signaling pathway for survival and proliferation. Depriving these cancers of arginine negatively regulates the mTOR pathway, and together with their deregulated cell cycling machinery, leads to cell death via various routes, including apoptosis, autophagy and necrosis.
Hepatocellular Carcinoma (HCC)
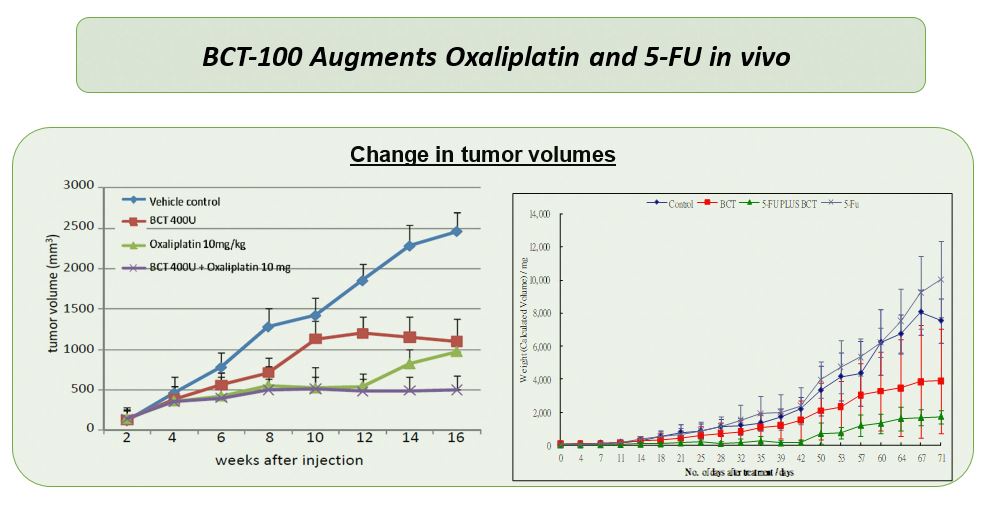
Anti-tumor efficacy of BCT-100 has been demonstrated in a number of tumor xenograft mouse models, both as single agent and in combination with chemotherapies. So far a phase I/II and two phase II clinical studies in advanced HCC were completed, either as single agent (BCT-100-002, phase I/II in HK; BCT-100-006, phase II in HK) or in combination with chemotherapy (BCT-100-004, phase II in HK), and demonstrated favorable safety profile and indication of survival benefit.
Acute Myeloid Leukemia
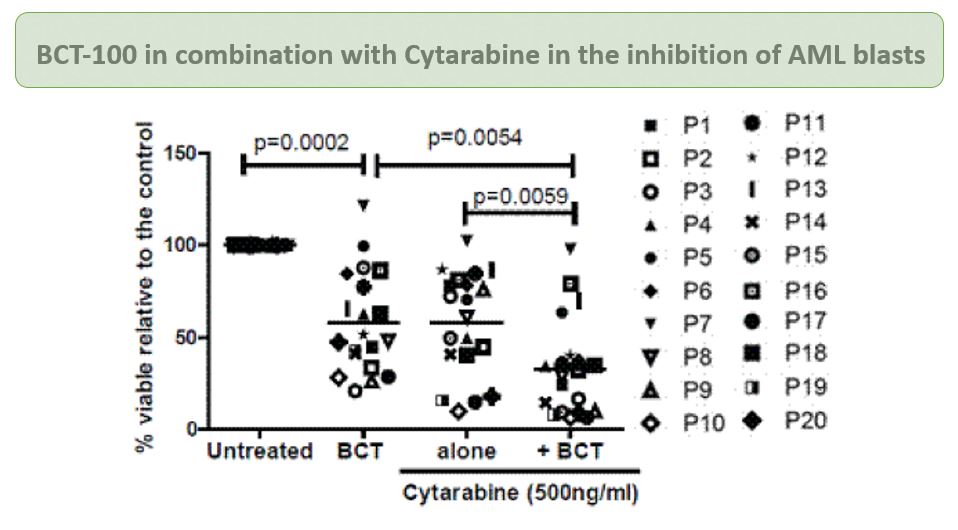
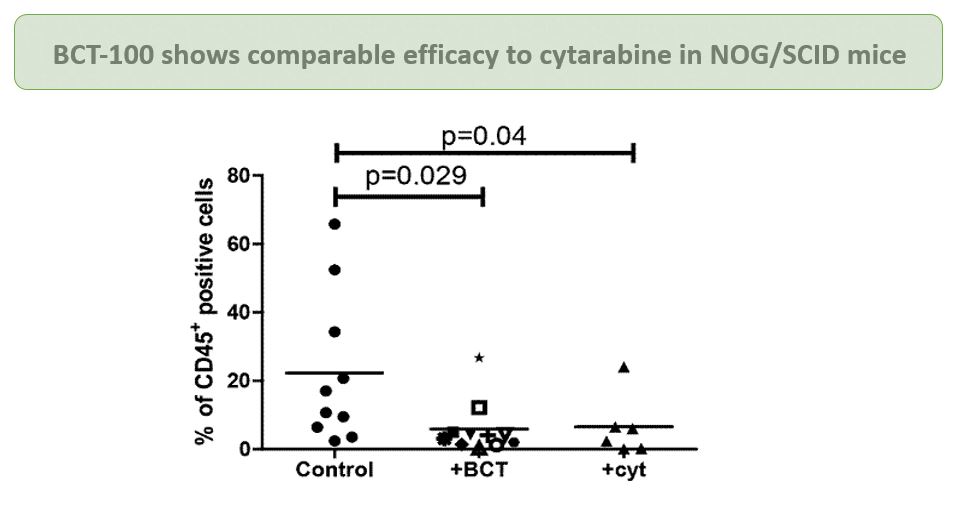
In vitro and in vivo studies indicated that BCT-100 treatment leads to a rapid depletion in extracellular and intracellular arginine concentrations, resulting in arrest of AML blast proliferation and AML engraftment. BCT-100 has been shown to act synergistically in combination with cytarabine, a standard treatment for AML patients.
Currently, a Phase II trial is being conducted in patients with relapsed or refractory AML in the UK.
Pediatric Cancers (Sarcomas, Leukemia, High-grade Glioma, Neuroblastoma)
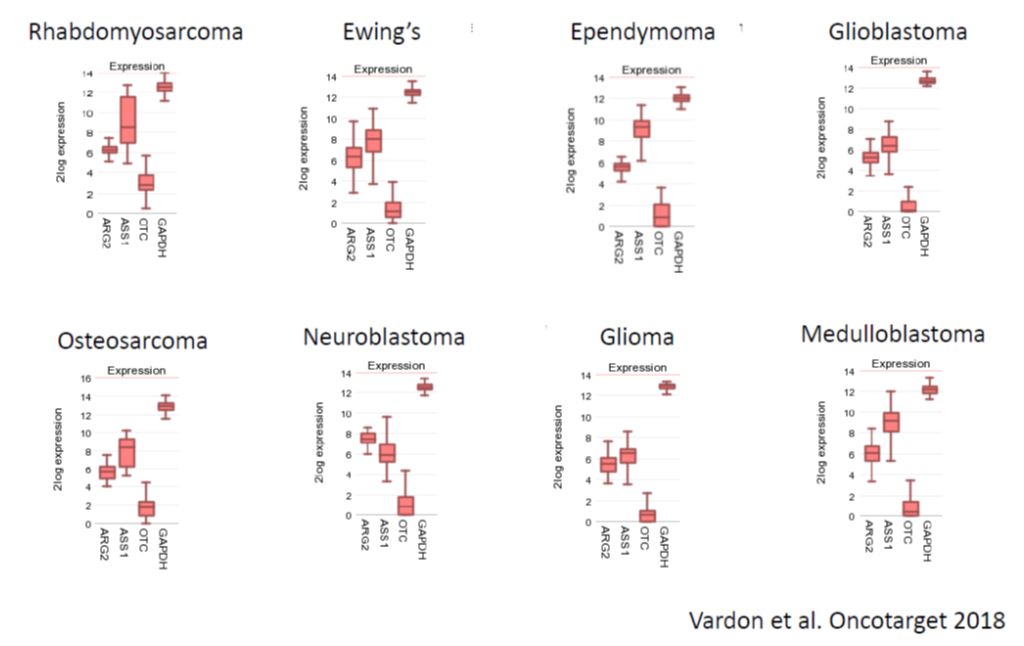
Young patients with relapsed/refractory leukemia, neuroblastoma, sarcoma and high-grade gliomas (brain cancers) have very limited therapeutic options that are plagued by significant toxicity and generally poor treatment outcome. Newer and safer options complementary or alternative to standard chemotherapy have been long sought. Pre-clinical studies have shown that these pediatric cancers are arginine auxotrophic. BCT-100 is now being evaluated as a novel, safe treatment regimen in a multinational clinical trial, PARC, which spans across the UK, Ireland and Australia. Thus far, favorable responses have been observed in pediatric patients heavily pretreated with and resistant to chemotherapy.
Melanoma
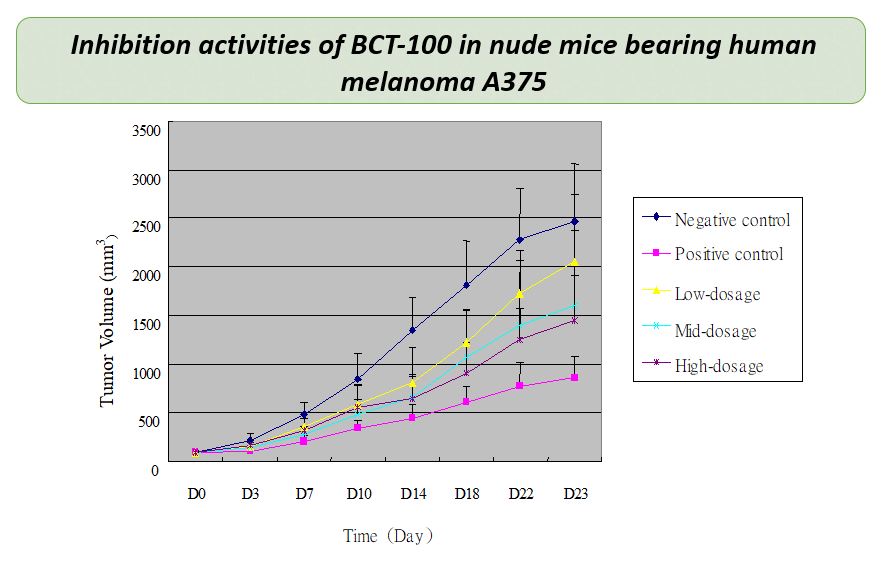
The advent of immunotherapy, as well as the discovery of kinase inhibitors for BRAF and MEK pathways, have been the major breakthroughs in managing high-stage melanoma. Yet, many patients received these treatments relapse within a few years. Alternative treatment strategy is still urgently needed. Melanoma is auxotrophic for arginine. Pre-clinical studies have shown that BCT-100 could inhibit tumor growth in melanoma A375 grafted murine model in a dose dependent manner.
BCT-100 is now being clinically tested as a treatment option for advanced melanoma and has demonstrated very promising therapeutic outcome. In particular, a patient with recurrent, metastatic melanoma who progressed through immunotherapies with two checkpoint inhibitors showed a durable complete response after being treated with BCT-100.
Biomarker-positive, arginine auxotrophic tumors
BCT has developed a panel of biomarkers that can indicate arginine auxotrophy at molecular level, as well as predicting and monitoring patients’ treatment response to BCT-100. This allows tailoring of medical treatment to individual patients. Biomarker-driven clinical trials have been planned to clinically verify and to further optimize the panel as companion diagnostic (CDx).
BCT-100 in Immune System Disorders
In the inflammatory milieu, arginase 1 is highly expressed in infiltrating macrophages and neutrophils. The arginase expressed thus depletes arginine in the inflammatory milieu where infiltrating lymphocytes, mainly T cells, are in abundance in response to inflammation. Arginase acts as a paracrine molecule to counterbalance the T cell responses to inflammation by suppressing their cytokine production and inducing T cell anergy, thus preventing progressive tissue damage.
BCT-100, given systemically, has been shown to reduce the severity of target organ damage and induce remissions in experimentally induced multiple sclerosis and rheumatoid arthritis. It has also been demonstrated that BCT-100 is effective in reducing graft-versus-host disease and lethality by suppressing T cell functions.
For an in-depth scientific review of these studies, please check out our scientific publications.